Aging Barriers and Endothelial Senescence
How Does Endothelial Senescence Modulate Organ Interfaces in Aging and Disease?
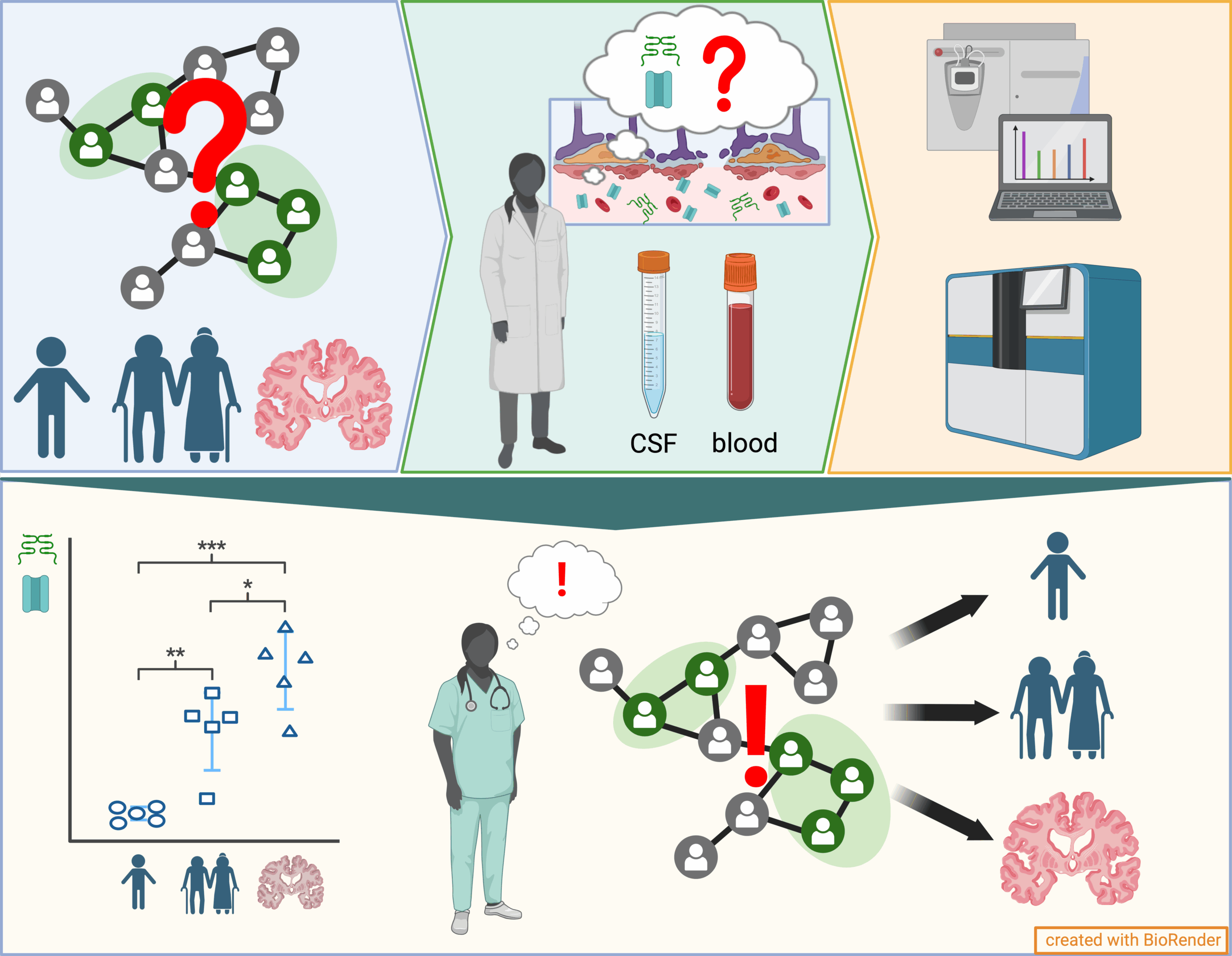
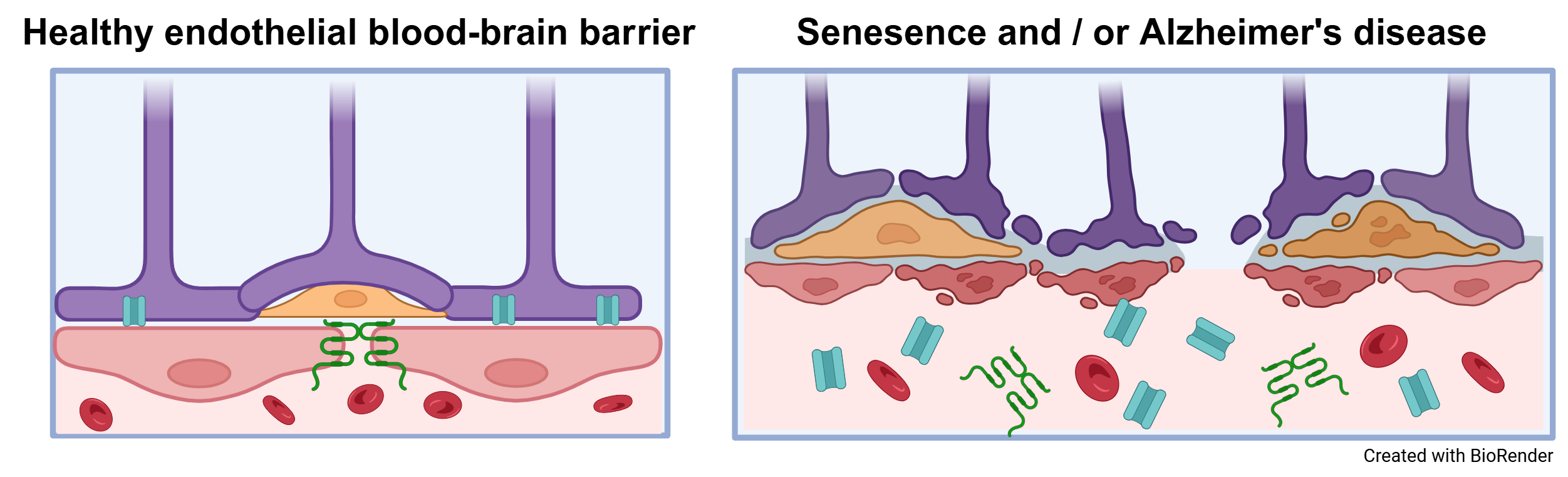
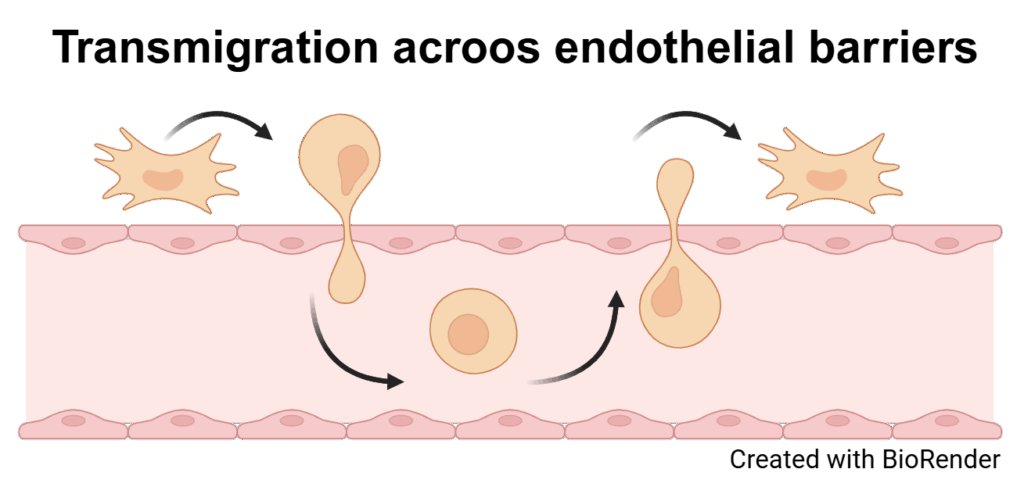
Endothelial cells (ECs) form highly specialized, selective barriers between organs and the vascular system and are essential for maintaining physiological processes and protecting organs from harmful influences. These barriers allow the controlled exchange of substances, ensuring that only specific molecules can reach the organs. With increasing age and in the course of diseases—particularly as a result of cellular senescence, which is an irreversible arrest of the cell cycle caused by internal or external damage—these complex barriers can become dysfunctional. So far, little is known about how senescence directly affects the function and integrity of endothelial barriers such as the blood-brain barrier (BBB) and peripheral blood vessels. The aim of this interdisciplinary research project is therefore to systematically investigate the consequences of senescence on endothelial barriers in the context of aging processes and diseases such as dementia. The approach combines innovative in vitro BBB models, derived from human induced pluripotent stem cells (iPSCs) of donors with different ages and health backgrounds, with in vivo animal models that allow for the analysis of age- and senescence-associated changes in the peripheral vascular system. Particular attention is paid to the role of sialic acids as key a molecule in the regulation of barrier function, along with specific transmigration experiments to evaluate the integrity of these barriers. In parallel, blood samples from cohorts of patients with neurodegenerative diseases are analyzed for senescence-associated biomarkers, aiming to identify new diagnostic markers and therapeutic approaches. This multidisciplinary project draws on the combined expertise of medicine and life sciences at Martin Luther University Halle-Wittenberg, with the long-term goal of establishing a sustainable translational research network on cellular senescence and endothelial barriers funded by third parties.
Projektbeschreibungen
People
Dr. rer. nat.
Matthias Jung
Sprecher & SP 2 – PI
Physiologische Chemie, UMH
Dr. med.
Annemarie Thäle
SP 1 – PI
Neurologie, UMH
Dr. troph.
Juliane-Susanne Jung
SP 5 – PI
Anatomie & Zellbiologie, UMH
Prof. Dr. med.
Markus Otto
SP 1 – Co-PI
Neurologie, UMH
Dr. rer. nat.
Kaya Bork
SP 4 – Co-PI
Physiologische Chemie, UMH
Prof. Dr. nat.
Thomas Hollemann
SP 2 – Co-PI
Physiologische Chemie, UMH
Prof. Dr. med. Dr. rer. nat.
Claudia Grossmann
SP 3 – PI
Julius-Bernstein-Institut, UMH
Dr. rer. nat.
Astrid Gesper
SP 4 – PI
Physiologische Chemie, UMH